Decaf Coffee deconstructed
Share
Decaffeinated Coffee.
Some people frown upon it, some downright ridicule it but decaf is a very important product. There are many people (like me) whose sleep is affected by consuming caffeine in the late afternoons or early evening.
Other people are downright caffeine intolerant.
Decaf coffee can be made using a number of different processes.
1. The Methyl Chloride(MC) process:
This is the oldest(and cheapest) process used to remove the caffeine from coffee. The coffee beans is steamed to open up the pores of the beans to facilitate
The decaf we sell are made using the CO2 process where liquid Carbon Dioxide is used to remove the caffeine. This process is chemical free and substantially more expensive than the MC process.
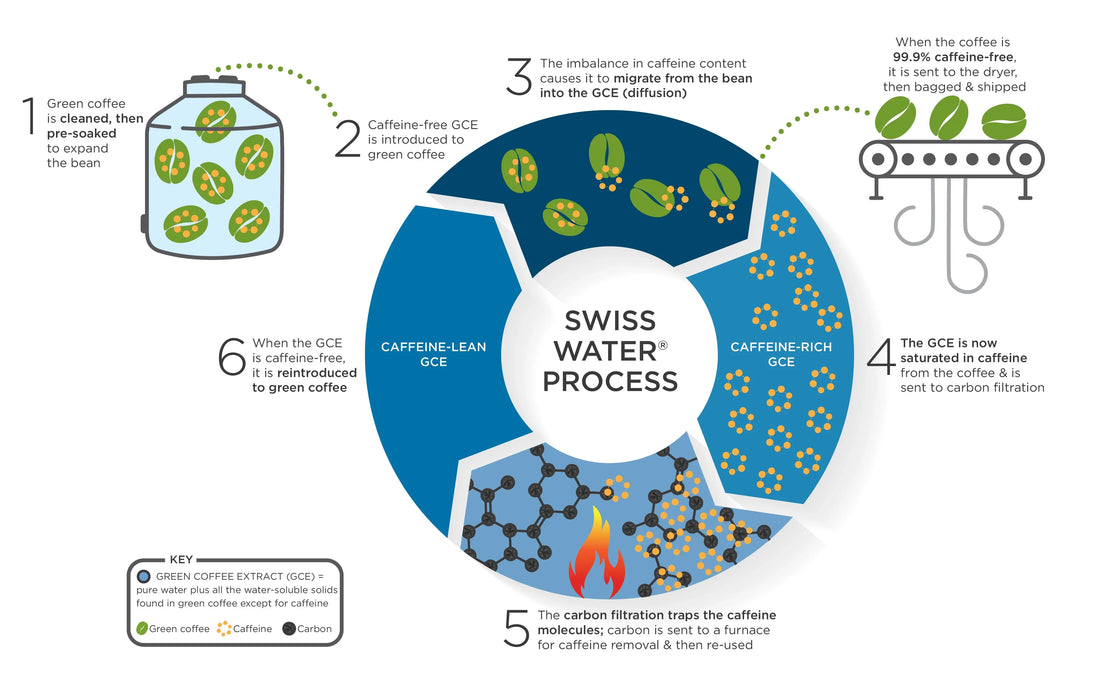
Then there is the water process which sounds simple but is quite complex.
A process I did not know off until very recently is the Sugar Cane process which is used widely in Brazil
Read more about it below:
As you know, the chemical caffeine is naturally found in the coffee bean, like in cocoa beans and in certain teas. To decaffeinate coffee, the caffeine must be physically removed from the beans. Caffeine is water soluble, so this is done in various methods of soaking coffee beans in water and a solvent that will separate the caffeine from the beans. The most difficult challenge is trying to remove the caffeine while preserving the rich, natural flavours of the coffee itself. Traditional decaffeinating processes used the water solvent method with the addition of heat or pressure. Adding too much heat or pressure forces the beans to swell to a great extent and may radically disrupt a green bean’s cellular structure, which ultimately degrades the final taste.
The Sugar Cane Process(aka ethyl acetate process), which originates in Colombia where sugar cane grows in abundance, avoids disrupting the bean’s cellular structure and even enhances sweetness of the final cup! To begin, fermented molasses derived from sugar cane is used to make ethanol. The alcohol ethanol is mixed with a natural acetic acid, to create the solvent ethyl acetate (E.A.). E.A. is also prominent in wine, beer, fruit, and vegetables. The coffee beans are soaked in water, which increases the bean moisture content and releases caffeine from the structure. After soaking for a sufficient period of time, the beans undergo an E.A. wash, which dissolves the caffeine. Finally, the beans are cleaned with water again, exposed to steam briefly to clean the inner portions of the bean, and then dried to the original moisture level. This process removes roughly 97% of the caffeine content. Due to the fermented molasses from the sugar cane, the Sugar Cane Process creates beans with a pleasant, clean, and sweet flavor!

